𝗠𝗘𝗗𝘄𝗶𝘀𝗲𝗣𝗞
June 20, 2025 at 08:09 PM
> 𝗠𝗢𝗦𝗧 𝗘𝗔𝗦𝗬 𝗪𝗔𝗬 𝗧𝗢 𝗥𝗘𝗠𝗘𝗠𝗕𝗘𝗥 𝗨𝗡𝗣𝗔𝗜𝗥𝗘𝗗 𝗘𝗟𝗘𝗖𝗧𝗥𝗢𝗡𝗦 𝗔𝗡𝗗 𝗗 𝗢𝗥𝗕𝗜𝗔𝗧𝗔𝗟 😁🌚✨
𝐇𝐎𝐖 𝐓𝐎 𝐃𝐑𝐀𝐖...?
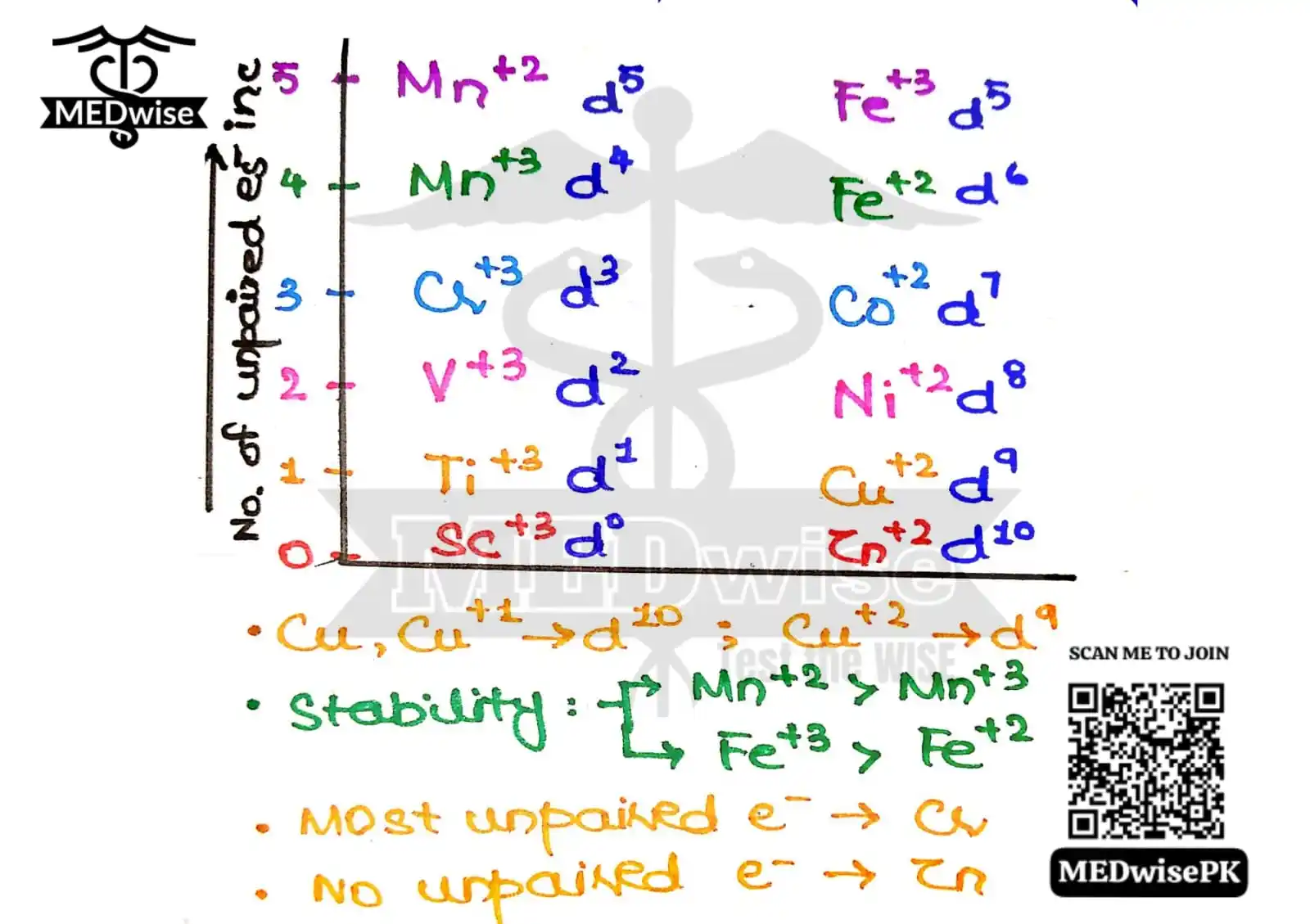
1.Write 0-5 counting on y axis(this 'll represent no. Of unpaired electrons).
2.Write Sc - Mn in +3 oxidation state from 0-4 in upward direction nd then Fe-Zn in +2 oxidation state from 4-0 in downward direction.
3.Write Mn and Fe again in front of 5 but this time; Mn with +2 & Fe with +3 oxidation state.
4.Also write d orbitals from d⁰ to d⁵ upwards then d⁵ to d¹⁰ downwards...
𝐍𝐎𝐖 𝐘𝐔𝐇 𝐂𝐀𝐍 𝐄𝐀𝐒𝐈𝐋𝐘 𝐓𝐄𝐋𝐋 𝐓𝐇𝐄 𝐍𝐎. 𝐎𝐅 𝐔𝐍𝐏𝐀𝐈𝐑𝐄𝐃 𝐄𝐋𝐄𝐂𝐓𝐑𝐎𝐍𝐒 & 𝐓𝐎𝐓𝐀𝐋 𝐄𝐋𝐄𝐂𝐓𝐑𝐎𝐍𝐒 𝐈𝐍 𝐃 𝐎𝐑𝐁𝐈𝐓𝐀𝐋 𝐎𝐅 3𝐝-𝐬𝐞𝐫𝐢𝐞𝐬 𝐖𝐈𝐓𝐇𝐎𝐔𝐓 𝐃𝐑𝐀𝐖𝐈𝐍𝐆 𝐂𝐎𝐍𝐅𝐈𝐆𝐔𝐑𝐀𝐓𝐈𝐎𝐍♾️🌾
> `ᴇɴɪɢᴍᴀ`
❤️
❤
3